Background: Allogeneic hematopoietic stem-cell transplantation has curative potential for sickle cell disease (SCD). Event-free survival (EFS) in children with SCD is >90% after a bone marrow transplant (BMT) from a myeloablative matched sibling donor (MSD). Unfortunately, <15% of patients with SCD have MSD, and myeloablative conditioning can be prohibitively toxic in adults with SCD. Reduced intensity HLA-haploidentical BMT with post-transplant cyclophosphamide (PTCy) has been shown in small studies to expand the donor pool with encouraging results. Still, concerns about graft failure and graft-versus-host disease (GVHD) persist. We present the results of a Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1507, multi-center single-arm, phase-II, prospective clinical trial (clinicaltrials.gov #NCT03263559) of haploidentical-BMT with PTCy to estimate EFS at 2-years in adults with severe SCD. Pediatric stratum data is not included in the data presentation and will be available in 2-years.
Study Design and Methods:
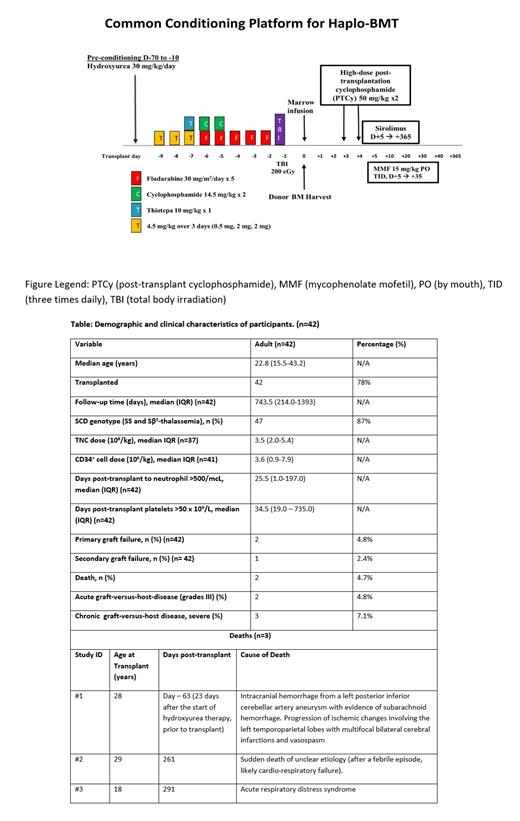
Eligibility: SCD patients aged 15.00-45.99 years with prior stroke, recurrent ACS or pain, chronic transfusion regimen, or tricuspid valve regurgitant jet velocity (TRJV) ≥2.7 m/sec were eligible. Participants were required to have an HLA-haploidentical first-degree relative donor, willing and able to donate bone marrow. The primary objective was EFS (survival without primary or secondary graft failure or second infusion of stem cells) at 2 years after haploidentical-BMT. Secondary objectives included determining the impact on clinical and laboratory manifestations of SCD and other transplant outcomes at 2 years post haploidentical-BMT. The protocol was opened for enrollment on 10/5/2017, completed accrual on 01/6/2021, and data are current as of 8/2023. Preconditioning with hydroxyurea (HU) 30mg/kg/day (Day-70 to Day-10); Conditioning regimen included Thymoglobulin (rATG), Thiotepa, Fludarabine, Cyclophosphamide, and Total Body Irradiation (TBI). GVHD prophylaxis included PTCy, sirolimus, and mycophenolate mofetil, figure.
Results: A total of 54 eligible participants enrolled from 19 sites; 42 (78%) proceeded to transplant. Amongst enrolled participants, 59.3% are male, 92.6% are Black, and 3.7% are Hispanic. 10 participants started HU but did not proceed to BMT, and 2 did not start HU or proceed to BMT. Reasons included donor issues (n=4), withdrawal of consent (n=2), insurance coverage (n=2), death (n=1), and other (n=3). 38/42 (90%) participants completed the study as planned; 2 participants withdrew consent, and 2 were lost to follow-up. The median age was 22.8 years at enrollment; 47/54 (87%) of enrolled participants had Hemoglobin SS disease, 40/54 (74.1%) had a Lansky/Karnofsky score of 90-100 at baseline, and 41/54 (75.9%) had an HLA match score of 4/8. Recurrent vaso-occlusive pain episodes (38.9%), acute chest syndrome (16.8%), and overt stroke (16.7%) were the most common indications for transplant. Only 13 (31%) participants achieved the intended 30 mg/kg/day dosing of HU preconditioning.
Estimated 2-year EFS is 88% (95% CI: 73.5%, 94.8%); all except one qualifying event occurred within 12 months. The 2-year overall survival (OS) post-HU was 93.0% (95% CI: 79.8%, 97.7%), and the 2-year OS post-transplant was 95.0% (95% CI: 81.5%, 98.7%); 2 (4.8%) participants had primary graft failure, and 1 (2.4%) had secondary graft failure before day +100. The cumulative incidence of grades II-IV acute GVHD at day 100 was 26.2% (95% CI: 14.0%, 40.2%), and grades III-IV acute GVHD at day 100 was 4.8% (95% CI: 0.9%, 14.4%). There were two deaths in the first year post-BMT (1 -organ failure; 1-ARDS), none in the second year; 33 (78.6%) participants reported at least one re-admission post-BMT, mainly due to either bacterial infection (n=41) or viral reactivation (n=36), table.
Conclusion: This multi-center phase-II trial of a reduced intensity haploidentical-BMT in adults with SCD shows durable donor engraftment at 2-years with low mortality. The 2-year EFS and OS are comparable to that reported after MSD myeloablative BMT. These results support haploidentical BMT with PTCy as a suitable and tolerable curative therapy for adults with SCD and severe end-organ toxicity such as stroke and pulmonary hypertension, a population typically excluded from participating in myeloablative gene therapy and gene editing trials.
Disclosures
Walters:AllCells, Inc: Consultancy, Other: Medical Director; BioChip Labs: Consultancy, Other: Medical Director; Vertex Pharmaceuticals: Consultancy; Ensoma, Inc: Consultancy. Solh:Bristol-Myers Squibb: Speakers Bureau. McKinney:Bluebird Bio: Other: Advisory Board; Horizon Therapeutics: Other: Advisory Board. Shaughnessy:Autolus Therapeutics, BMS: Honoraria; BMS, Sanofi: Speakers Bureau. Kanter:Novartis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; OptumRx: Consultancy; Beam: Consultancy, Honoraria; ECOR1: Consultancy; Fulcrum: Consultancy; Guidepoint Global: Consultancy; Watkins, Lourie, Roll&Chance: Consultancy; Bausch: Honoraria; Austin Therapeutics: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Chiesi: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Bluebird Bio: Consultancy; GLG: Consultancy; Cowen: Consultancy. Bollard:Roche: Consultancy; Cabaletta Bio, Catamaran Bio: Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity’s Board of Directors or advisory committees, Patents & Royalties: Patent applications in CAR-NKs. Cooke:Jazz Pharmaceuticals: Consultancy. Hanna:Sanofi: Speakers Bureau; SOBI: Speakers Bureau; Vertex: Honoraria; EDITAS: Research Funding. Mahadeo:Jazz: Honoraria, Research Funding. DeBaun:Novartis, Forma, Vertex: Consultancy, Other: Consulting. Brodsky:Alexion, AstraZeneca Rare Disease: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal